A second promising Covid-19 vaccine has been announced after its preliminary data showed good results.
US biotech firm Moderna claims its new vaccine is 94.5% effective. This comes less than a week after German firm Pfizer said its vaccine was 90% effective. Both of these results raise hopes that a range of immunizations will soon be available to help aid and eventually end the global pandemic that has been affecting people across the globe since the start of the year.
How effective is it really?
Though the statistics provided by Moderna declare that their vaccine is 94.5% effective, scientists who were not involved with the testing say they were encouraged by the results but caution that the FDA must scrutinise the data and decide if the vaccines can be used outside of a research study.
‘We’re not to the finish line yet’, said Dr. James Cutrell, infectious-disease expert from UT Southwestern Medical Centre in Dallas.
‘If there’s an impression or perception that there’s just a rubber stamp, or due diligence wasn’t done to look at the data, that could weaken public confidence.’
So, whilst the preliminary data is encouragingly effective, there is still a lot of room for hesitation.
What is still uncertain about the vaccination?
Aside from the effectiveness still being speculated, it is also still unknown how long immunity against Covid-19 lasts. For this to be known, the volunteers who have already had the vaccination administered to them, will have to be monitored for a much longer period of time than they currently have been.
Indeed, it is also unknown whether people who have had the vaccination are still capable of spreading the virus. This is again another factor that can only be addressed if participants in the preliminary tests are monitored for a longer period of time.
How does the Moderna vaccination work?
Like Pfizer, the Moderna uses technology to make the vaccine based on a molecule known as mRNA. This molecule contains genetic instructions for making proteins inside cells. For this vaccination, researchers created an mRNA with the instructions for making the coronavirus spike protein – the protein that is the key to the virus infecting cells.
This protein is also what can trigger someone’s immune system to make the correct antibodies against the virus without causing an infection.
The vaccine undergoes a series of phases before it can be approved and made accessible to the public:
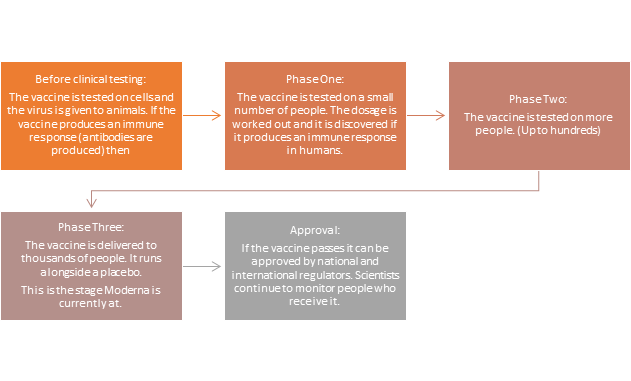
The trial involved 30,000 people in the United States: half were given two doses of the vaccine, four weeks apart, and the rest had dummy injections. The results analysis was based on the first 95 people to develop Covid-19 symptoms. Only 5 of the Covid cases were in people who had received the vaccine, and 90 were in those who were given the dummy vaccine.
The data also showed that there were 11 severe cases of Covid in the trial but they did not happen to people who were immunised.
The data safety and monitoring board did not identify ‘any significant safety concerns’ regarding the side effects of the vaccine. They included pain at the site of injection, fatigue, and aching muscles/ joints, just as can occur after other vaccinations.
When will it be made available to the public?
It has been predicted that more than 1 billion people could be immunised against coronavirus by the end of next year.
Matt Hancock announced that the UK has signed an ‘initial agreement’ to purchase five million doses from April next year. This would treat 2.5 million people.
However, he emphasised that there is no ‘stockpile’ of these vaccines as they are yet to be manufactured. This is why Europe will not receive its first batch until April next year.
Moderna says that is will be applying to regulators in the United States in the coming weeks, hoping to have 20 million doses available in the country. It is understood that the Moderna vaccine may not be widely available in Britain because US citizens are being given priority.
How do the Pfizer and Moderna vaccines compare?
Given that both vaccines have been announced in the last week, you would be forgiven if you are hazy on the distinction between the two vaccinations.
The chart below summarises some of the main differences between the two:
Data from: https://www.bbc.co.uk/news/health-54902908
The work put into producing this vaccine at such a speed is an achievement in itself, with results being, as Dr. Fauci said, ‘truly striking’. It is an attempt not only to get the science right but to find some ‘light at the end of the tunnel.’
Written by Amelia Cutting
Featured image courtesy of Pixxl Teufel via Pixabay. Image license can be found here. This image has in no way been altered.
In-text graphics courtesy of article author, Amelia Cutting.

